The 2020 coronavirus pandemic has highlighted more than ever the importance of drug repurposing in new pharmaceutical product innovation.
The point is obvious: repurposing offers a route to finding a new treatment rapidly, allowing introduction into the clinic and bypassing much of the drug discovery process. One of Numedicus’ associated companies is developing a repurposed drug for COVID-19.
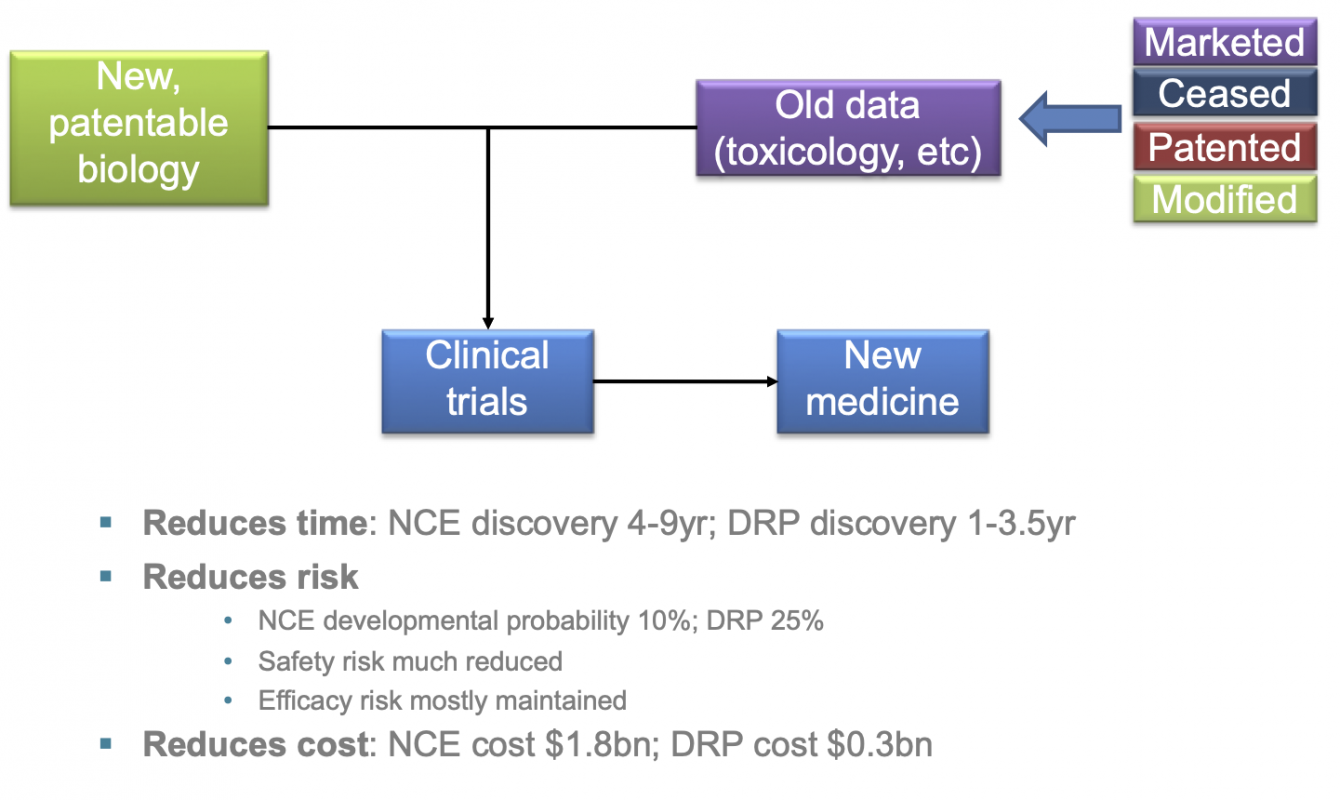
But there are two other important aspects of repurposing to highlight:
- probability of success
- cost of innovation
The chances of toxicological failure are much less with a drug that has already been through the basic toxicological tests needed for first-in-human studies. Conversely, the probablility of clinical success is much higher.
And, as a result of the shortened timelines to commercialisaiton together with reduced risk of failure, it is estimated that new repurposed medicines can be identified at around a third to a quarter of the costs for a new chemical entity.
Pharmaceutical prices are becoming unbearable for even the best resourced healthcare systems in the world, like the USA. The increased proportion of biological medicines, and their long exclusivity times makes this a problem that will deepen, and drug repurposing offers a fundamental means to address this challenge.
In December 2019, the FDA and NCATS held a workshop to address the regulatory and developmental challenges to the repurposing of off-patent medicines. Change is coming!